Enzyme Active Site and Substrate Specificity
A substance that helps a chemical reaction to occur is a catalyst, and the special molecules that catalyze biochemical reactions are called enzymes. Almost all enzymes are proteins, made up of chains of amino acids, and they perform the critical task of lowering the amount of energy needed for the chemical reaction to take place inside the cell. Enzymes do this by binding to the reactant molecules, and holding them in such a way as to make the chemical bond-breaking and bond-forming processes take place more readily.
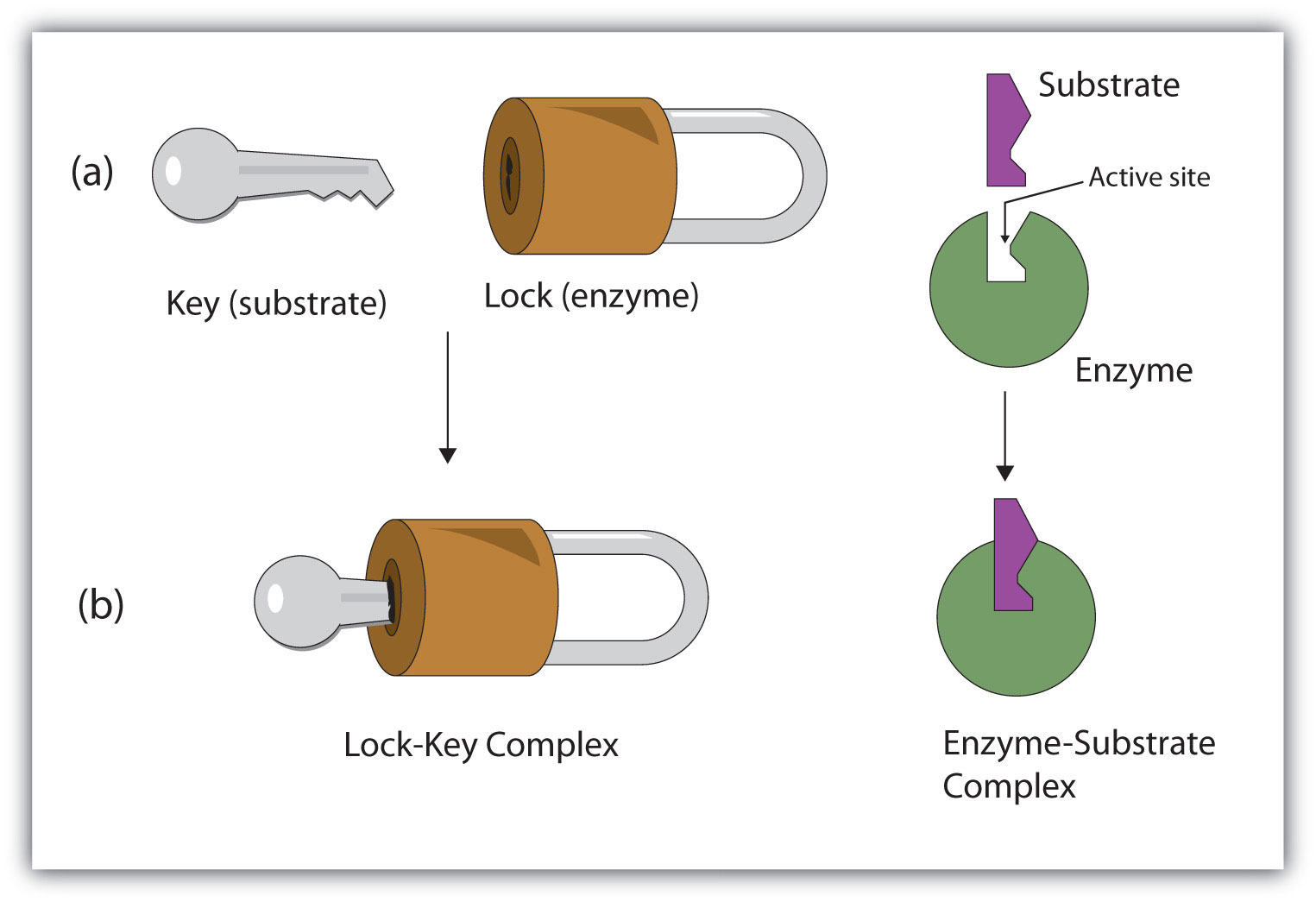
The chemical reactants to which an enzyme binds are the enzyme’s substrates. There may be one or more substrates, depending on the particular chemical reaction. In some reactions, a single-reactant substrate is broken down into multiple products. In others, two substrates may come together to create one larger molecule. Two reactants might also enter a reaction, both become modified, and leave the reaction as two products. The location within the enzyme where the substrate binds is called the enzyme’s active site. The active site is where the “action” happens, so to speak. Since enzymes are proteins, there is a unique combination of amino acids in the enzyme's active site. The unique combination of amino acid, their positions, sequences, structures, and properties, creates a very specific chemical environment within the active site. This specific environment is suited to bind to a specific chemical substrate (or substrates). Due to this jigsaw puzzle-like match between an enzyme and its substrates, enzymes are known for their specificity. The “best fit” results from the shape and the attraction to the substrate of the amino acids in the active site . There is a specifically matched enzyme for each substrate and, thus, for each chemical reaction. Each enzyme will work with only one type of substrate. This is called the "lock and key" hypothesis.


The fact that active sites are so perfectly suited to provide specific environmental conditions also means that they are subject to influences by the local environment. This means that changing the local pH or temperature can change the nature of the active site, changing how it is attracted to the substrate. Increasing or decreasing the temperature outside of an optimal range can affect chemical bonds within the active site in such a way that they are less well suited to bind substrates. High temperatures will eventually cause enzymes, like other biological molecules, to denature, a process that changes the natural properties of a substance. Likewise, the pH of the local environment can also affect enzyme function. Active site amino acid residues have their own acidic or basic properties that are optimal for catalysis. These residues are sensitive to changes in pH that can impair the way substrate molecules bind. Enzymes are suited to function best within a certain pH range, and, as with temperature, extreme pH values (acidic or basic) of the environment can cause enzymes to denature.
Section Summary
Enzymes are chemical catalysts that accelerate chemical reactions at physiological temperatures by lowering their activation energy. Enzymes are usually proteins consisting of one or more polypeptide chains. Enzymes have an active site that provides a unique chemical environment, made up of certain amino acids. This unique environment is perfectly suited to convert particular chemical reactants for that enzyme, called substrates, into unstable intermediates called transition states. Enzymes and substrates are thought to bind with lock and key fit, which means that enzyme's active site matches with a specific substrate. Enzymes bind to substrates and catalyze reactions in four different ways: bringing substrates together in an optimal orientation, compromising the bond structures of substrates so that bonds can be more easily broken, providing optimal environmental conditions for a reaction to occur, or participating directly in their chemical reaction by forming transient covalent bonds with the substrates.
This text is adapted under a Creative Commons 4.0 license. You can find the original source here.
Environmental factors influence enzyme activity
The fact that active sites are so perfectly suited to provide specific environmental conditions also means that they are subject to influences by the local environment. The environmental conditions can include temperature, pH, salinity and presence of heavy metals. It is true that increasing the environmental temperature generally increases reaction rates, enzyme-catalyzed or otherwise. However, increasing or decreasing the temperature outside of an optimal range can affect chemical bonds within the active site in such a way that they are less well suited to bind substrates. High temperatures will eventually cause enzymes, like other biological molecules, to denature, a process that changes the natural properties of a substance. Likewise, the pH of the local environment can also affect enzyme function. Active site amino acids have their own acidic or basic properties that are optimal for catalysis. These amino acids are sensitive to changes in pH that can impair the way substrate molecules bind. Enzymes are suited to function best within a certain pH range, and, as with temperature, extreme pH values (acidic or basic) of the environment can cause enzymes to denature an the rates of the reaction decrease.
The enzymes in your body are also most functional at specific pH levels. In the stomach there is a high acidity due to the hydrochloric acid used to break down food and kill bacteria. As a result, only protease (the enzymes that break down proteins) are functional in the stomach as they have an optimum pH of about 2. Amylase, which is functional in the mouth and small intestine works at a much higher pH, one much closer to 6.
CLick here to try changing the concentration of a substrate and see the effect of this on the reaction rate.
CLICK HERE FOR THE VIRTUAL ENZYME LAB


No comments:
Post a Comment